Axel Hoos, MD, PhD
Head, Therapeutic Area Oncology & Immuno-Oncology
GlaxoSmithKline
“This is a nut we can crack.”
- Hoos
Axel Hoos was born in 1969 and grew up surrounded by the gentle hills and forests of Germany’s state of Hessen, in a village called Sondheim. Population: roughly 200 souls.
“It’s a tiny town,” says Hoos, in a manner as crisp as his favored blue suits. The town was so small in fact that he spent much of his time growing up there in the surrounding forests—there were oaks, and birch, and there was Hoos, running, long-distance running which he did competitively and which remains his physical discipline to this day.
“As you train your ability to perform increases, you have much more capacity to process oxygen, muscles are functioning better, and you produce endorphins when you’re moving fast and that feels good,” says Hoos, pausing—private man that he is—reaching for the right word to describe what he’s probably not put into words before. “You… you feel like you’re flying. It detaches you a bit from reality—you can just live the performance.”
As a young man he ran/flew through the forests of Sondheim until one day he realized he had to leave. He wanted to do big things. He wanted to make a difference. He couldn’t do that in Sondheim. “So I left.” Hoos’ brevity of comment masks his feelings for the town and for the quiet of the forest—save for his footfalls. Not one to wear his passions on his sleeve, rather, Hoos is armored by clarity of purpose.
This demeanor is critical to the story of ipi: James Allison was the wily discoverer; Jedd Wolchok the clinical pioneer; Axel Hoos: the indomitable drug developer and corporate champion.
Making of a Champion
“In high school my favorite subject was philosophy,” recalls Hoos, “And German—so literature.” The draw for both was the same—there was a narrative, and Hoos likes a good story. However, the German school system requires a diversity of interests in their students so Hoos chose to take biology along-side his core classes. It was a prescient pick.
“It’s interesting—one of the things that excites me in philosophy and literature is stories, human stories, and I quickly found out that science has them too,” Hoos says. “You get all kinds of crazy stories—Watson and Crick and the double helix for example—that one really stuck with me.” The most important take away from the book, according to Hoos, was Watson’s conviction. “If you believe in the science that you’re doing, and you think you can have an impact you won’t be easily disturbed from that by a non-believer—and there are always more of those than not—trying to talk you out of it. And that lesson clearly applies to immuno-oncology. So I got excited about science through these stories that science tells.”
Thus, a biology-major was born.
But it wasn’t enough. Learning for the sake of learning lacked punch; Hoos wanted to make a difference. “I’m always looking to move something, to make an impact.” Biology was fine, it’s the stuff of life after all, but the stuff of basic science is generally not geared towards utility. It’s not translational work. If you want to make a notable mark in the near-term, reasoned Hoos, you take the biology into medicine. So that’s what he did, he chose to be a physician, obtaining his degree from the prestigious Heidelberg University. (Another prescient choice.)
“On the same campus where Heidelberg University is situated there’s a place called the German Cancer Research Center (DKFZ, in German),” Hoos explains. “It’s one of the largest cancer centers in Europe, with academic science and academic medicine, so I chose to do my Ph.D. there.” (Combined MD/PhD programs do not exist in Germany. Studies are completed independently.)
Next into the mix in the making of Dr. Hoos was the choice of Ph.D. thesis. “I got fascinated with molecular biology,” he says—the underpinnings of regular biology. The extra classes with a molecular slant were both a benefit and bane: it meant a great deal more work.
“It sparked my interest in a thesis that would be more exciting than a superficial science project.” So Hoos took his enthusiasm and popped in next door at the DKFZ, identifying as his thesis the elucidation of immune regulation by human papillomavirus.
It was a cunning choice. “It was a strong topic at the DKFZ, because the director of the center at that time was Harald zur Hausen.” Dr. zur Hausen would go on to receive a Nobel Prize for his work on papilloma viruses in 2008.
Dr. Hoos’ thesis work took three years to complete while he attended medical school at the same time. To sum up: “Basically, a lot of nights and weekends. Little personal time.”
The Cutting Edge
All that science still wasn’t enough. “I realized I still needed to be a physician,” says Hoos, and there was an obvious, and profound unmet medical need right there waiting for him. “I had learned about cancer and I found it a challenging subject because we needed better treatments than giving out poison—because that’s what chemotherapy is.” So if you want to have direct impact on cancer what do you do? You cut it out.
“The simple solution for me was to become a surgeon and do science on the side.” For academics in Germany it is possible to do both—treat patients, and have a lab. At least, that was the plan. “It was a naïve thought. The surgery program is an all-absorbing thing. It eats you.”
Not willing to sacrifice the science, Hoos looked for another way, a way that presented itself in the form of a research grant from the German government that would cover a two-year stint at an international academic institution. The institution in the present case (drum roll please): Memorial Sloan Kettering Cancer Center, New York City.
The cutting edge indeed.
“I chose Alan Houghton’s lab (chapter xx)—tumor immunology.”
(One could almost have said: “Once upon a time…”)
“I was a fellow in Dr. Houghton’s lab (around 2000) at the same time as Jedd Wolchok (chapter 2); that’s how we know each other.”
The lab was a special place, run by an extraordinary individual who had been diagnosed with ALS before Hoos’ arrival.
“When I first came to the lab Dr. Houghton had already lost his ability to walk, but he was still able to speak,” recalls Hoos. “He had a wheelchair with a joystick—he saw patients, he directed patient care, and he continued to run the lab, running lab meetings, giving advice on how to design experiments… The strength of his mind and his character was so impressive – he inspired people.”
The work during Hoos’ fellowship focused on a technology colloquially referred to as a “gene gun”, whereby nano-scale gold particles coated with antigens are loaded into an air gun and “shot” into the patient’s skin; it’s a method of vaccination.
The work was fascinating. The work was innovative. The work was not enough. “I was still a few too many steps away from the patient,” says Hoos. To move closer he used his position within the department of surgery at MSKCC to bring scientists and surgeons together. “That’s the advantage I had being a surgeon in Alan Houghton’s lab. I had access to the entire clinical department.” Hoos began offering clinical scientists translational research projects, for instance, measuring biomarkers in a patient’s tumor that could be correlated with clinical outcomes and would possibly be useful for predicting outcomes, targeting new therapies or potentially help with treatment decisions.
[I was thinking here that it might be useful to explain what a biomarker is, given how important biomarkers have become for modern development in IO – PR]
“And that opened the door for many projects because it’s translational,” says Hoos. You can see the results in the patient. “And that excites everybody.” Taking this approach, Hoos and collaborators published 20 papers from the data generated in just two years.
And without realizing it, Hoos had assumed his first leadership role, with cross-discipline cooperation as its philosophical core.
“I didn’t view myself as a leader,” says Hoos. “I just did stuff that led to a result.”
The Dark Side
After that stellar two years the fellowship ended, and the question was called: Go back to Germany, or stay in the U.S.? Live in the clinic, or the lab? There was a proposal for another surgical residency, this time at Harvard no less… It was tempting but he wanted to help patients in other ways—he wanted to do research.
“My choice was ultimately to go into industry, because I thought I could make a greater impact,” and the reasoning was a bit naïve, but straightforward: “I didn’t know what industry was actually all about, I just knew that at the end of the (scientific) story, they are the ones that deliver the medicines.”
As luck would have it, Hoos’ direct supervisor at MSKCC was Jonathan Lewis, who had recently been offered the chief medical officer position at a Boston-based company called Antigenics. Dr. Hoos went with Dr. Lewis to Antigenics: Dr. Hoos went to The Dark Side. (This phrase if often used by academics to describe the pharmaceutical industry.) “’Dark side’, right? I sometimes jokingly use the same phrase, but I’ve never seen it as that.” What Hoos saw was merely a new path to deliver greater impact.
Of course, this view is not universally held. In any situation where huge sums of money are involved there will be tensions. There will be egos. And there will be attitudes. “From a personal perspective, my relationships (with academic investigators) remained unchanged,” says Hoos, but when engaging academics professionally he has witnessed institutional bias from some who behaved as if their contribution to the cause (bankrolled by biotech) was somehow above the corporate fray.
For the most part though, as one might expect, Hoos just cuts to the chase. “There are good and bad guys on both sides,” says Hoos, and the best and the worst have been evenly distributed. Seeing the world as otherwise is counterproductive. (It’s difficult to imagine that Axel Hoos, at any point in his life, did anything counterproductive.) “In today’s environment everything is collaborative—you need both sides coming together to be successful.”
The Road to Ipi
Hoos started at Antigenics with no prior background in industry, and no experience in drug development, “so I had a steep learning curve.” Antigenics had a product in development called Oncophage, a personalized vaccine approach using the patient’s own surgically resected tumor tissue as a basis for the treatment (it’s way more complicated than that but this book can only be so long).
“The approach made good scientific sense,” says Hoos, “And it was something that brought several worlds together—the worlds of surgery, molecular biology, immunology, and industry all packaged in one thing, and I liked that, and I learned a lot.”
And it didn’t work.
“It didn’t play out because at the time nobody had heard of checkpoint modulation,” which turned out to be a HUGE problem: Any inflammatory activity of T cells due to vaccines may be attenuated by an immune checkpoint—insufficient clinical activity. And there was another problem that wasn’t entirely clear at the time: The clinical activity patterns of IO therapeutics, i.e. vaccines, are different than that of chemotherapy or targeted agents.
“I was at Antigenics for about five years,” says Hoos, “And midway through it became very clear that the development paradigm we were using to develop a cancer vaccine by handling it like a chemotherapy does not make sense.”
To address the issue (proactive as ever) Hoos built the Cancer Immunotherapy Consortium in 2003, a non-profit dedicated to the systematic improvement of development problems of cancer immunotherapies. Its first task was the review of clinical endpoints for immunotherapies as observed by the clinicians using them. Originating from this years-long, cross-discipline, multicenter effort were a number of critical publications, one in particular written by Hoos in 2007 that, despite its focus on vaccines laid the foundation for a paradigm shift in clinical trial designs for all immune-therapeutics.(Hoos et al. 2007) Two years later the landmark position paper on immune-related response criteria—authored by Hoos, along with Jedd Wolchok—was published: Guidelines for the Evaluation of the Immune Therapy Activity in Solid Tumors: Immune-Response Criteria. (Wolchok et al. 2009)
The fledgling IO industry finally had some guidance, without which ipilimumab might never have been approved, and the immunotherapy approach might have once again slipped into the shadows.
Ugh
In the meantime, Oncophage failed and Hoos hit the pharmaceutical road.
“I had five years of experience in biotech,” says Hoos, ever determined. “I wanted to develop the drug. I wanted to get the drug over the finish line.” It didn’t happen. But Hoos was beginning to understand why. Reason one: Something was turning the activity of T cells off (checkpoint modulators had yet to be named); reason two: the response impact of drugs effecting the immune system cannot be easily measured using traditional means (thus the Consortium above); and three: small biotech did not have the required clout to do these trials. “I learned a good amount there, but I missed something. I needed the big pharma experience.”
Hoos moved to BMS.
BMS and Ipi
The early clinical trials with ipi had been reported (by Jedd Wolchok and others), and despite low response rates (5-10%) Dr. Hoos had a good feeling the drug mechanism would pan out to be central for clinical activity, though skepticism was everywhere. “Most people were thinking this was never going to work,” says Hoos, and besides, targeted therapies were the real magic bullet, everybody knew that, why bother with the impossibly complicated immune system?
This sense was endemic. A best-selling case in point, as pointed out by Hoos: The Emperor of all Maladies, published the year before ipi was approved contains not one word about cancer immunotherapy. “The information was out there saying that immunotherapy was doing something, but it was ignored. Mainstream oncologist didn’t believe in it.”
[I would say a few words about the book for context, the author, what it is about, etc. – seems like a throw-away reference without at least a simple description – PR]
But no matter. They believe in it now. And here’s why:
What BMS had in place at the time was a protocol designed by someone skilled in study of chemotherapy. It was inappropriate, and would have resulted in failure. “(That individual) put a chemotherapy plan in place,” says Hoos, but to be fair, he adds that all oncologists would have put a chemotherapy plan in place at that time. No one knew better.
But Hoos thought he could do better, and with no small amount of arm-twisting of the internal stakeholders (it took two years) aspects of the new trial design paradigm were implemented for ipi. “I probably wouldn’t have been successful on my own if Pfizer didn’t have tremelimumab, another CTLA-4 antibody in the clinic at the same time—so two CTLA-4 antibodies, both being tested in metastatic melanoma in Phase III programs at the same time.” It was a head-to-head race. One failed*. One didn’t. Why?
It was a matter of timing. “When Pfizer failed, BMS had to rethink what we were doing for the obvious reason – we could also fail,” says Hoos. “And if Pfizer failed with a standard approach and BMS had that standard approach…” Suddenly (after two years), “BMS reconsidered and started using my paradigm.”
This was 2008, the year after Hoos’ game-changing paper. Before 2008 good response rates, defined as tumor shrinkage, were taken as a sign what one might find on overall survival. As a general rule in Oncology, response benefits are usually greater than survival benefits. This is often due to the development of treatment resistance: patients respond only to eventually relapse and subsequently die.
[Because…? Maybe say a few words about why cancer is such a wily beast, you know, like the “whack-a-mole references – PR]
“Here it was the complete opposite,” says Hoos. “Response outcomes were small and survival outcome was big.” Some patients who did not respond tended to still do very well with survival, some, survived for years beyond their initial short-term, terminal prognosis. And some have been in remission so long as to be considered cured. “So we figured that we did not yet have validated tools to capture all benefit a patient can experience from immunotherapy. We therefore changed the primary endpoints of our Phase 3 trials to survival, which ultimately would capture the therapeutic benefit.”
Dark Night
“My plan was I want to have impact. And this is a nut that we could crack. So I stayed with it.”
Okay then.
Vindication
A single moment can settle a lifetime of arguments. It might be an apology. It might be a hug. It might be statistics.
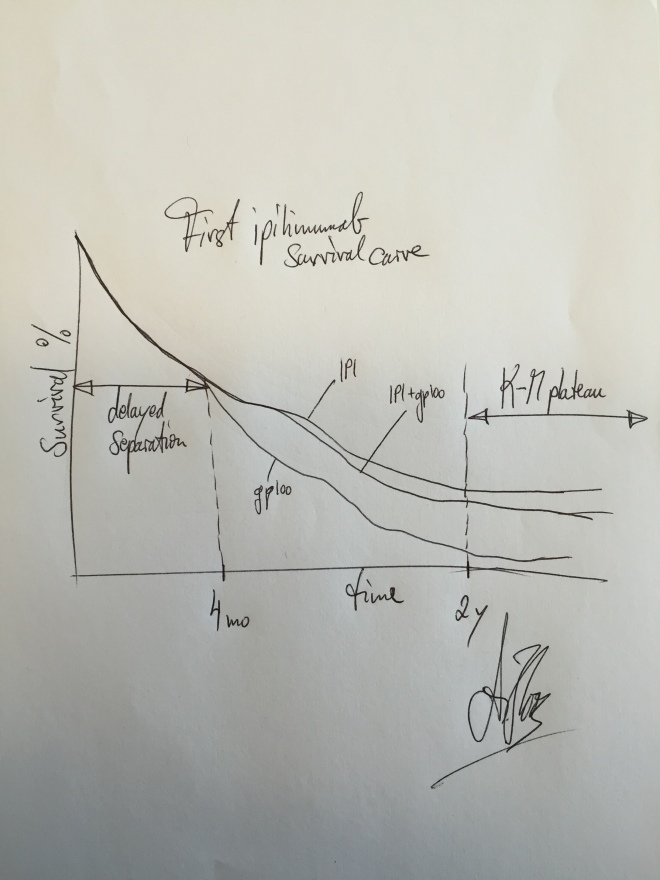
“The very first time I knew I had something big was when my statistician at BMS came to me and showed me the Phase III survival curve for ipilimumab,” recalls Hoos. “Mind you, this is the first person to ever see the data, and he pulls it off the printer and says, ‘Oh, by the way, here’s the survival curve from that trial.’” And he sets it down.
“I looked at the survival curve…” (He pauses, with half a lifetime of work filling the brief silence.) “I looked at the curve and I knew I had something.” The curves showed that, initially, patients on ipi and patients in the control arm had roughly equivalent survival for the first few months, but after more follow-up time,—far longer than you would expect with chemo—the two curves, one immunotherapy, one not, began to separate.
That was merely the good news. The great, the amazing, eyes-wide news was the so-called tail. For chemo, a survival curve starts with a line at the upper left corner of the graph—when all patients in the study are still alive—and then the line progresses downward to the right as patients on the study die off. Since very few cancer treatments are curative that line, if you wait long enough, eventually winds down to a surviving population of close to zero.
That’s not what Hoos was looking at. He was looking at a tail: a line that sloped ominously downward over two years as time progressed, but then plateaued, flattened out. Finito. The tail of the curve showed that roughly 20% of patients were experiencing long-term survival in excess of two years.
Hoos let it sink in, and then he knew: “Immunotherapy was no longer a hypothesis. This was an answer—a definitive answer. It’s working.”
On March 25, 2011, the U. S. Food and Drug Administration approved ipilimumab for the treatment of unresectable or metastatic melanoma.
The long distance runner had finished his finest race.
Wolchok, J. D., A. Hoos, S. O’day, J. S. Weber, O. Hamid, C. Lebbe, M. Maio, M. Binder, O. Bohnsack, G. Nichol, R. Humphrey, and F. S. Hodi. “Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria.” Clinical Cancer Research 15.23 (2009): 7412-420. Web.
Hoos, A. “A Clinical Development Paradigm for Cancer Vaccines and Related Biologics.” European Journal of Cancer Supplements 5.9 (2007): 5-7. Web.
*The development of tremilimumab has resumed under the sponsorship of AstraZeneca.